MEDICAL DEVICE DEPARTMENT
Contributing to Everyday Life and Health
The Medical Device Department was launched in 2018.
This new department includes the Medical Device Group within the head office, and carries out our activities with a license for manufacture and sales of class 2 medical devices, as well as a license for sales and lease of controlled medical devices. In addition, our Utsunomiya Plant, located in Utsunomiya, operates under a license for medical device manufacture.
The Medical Device Department applies Nihon Parkerizing’s surface treatment technology to medical devices, providing products that reduce burden on patients and enhance functionality for medical providers.
Quality Policy
In order to contribute to the realization of a fulfilling society through our medical devices, we:
- Ensure thorough compliance with laws and regulations.
- Provide safe products through our quality system and observance of rules and procedures.
- Carry out continuous improvements in order to provide products with further enhanced safety and value.
August 5, 2020
Mitsuru Matsumoto, Supervisor
Business Licenses
 Business License for Manufacture and Sales of Class 2 Medical Devices
Business License for Manufacture and Sales of Class 2 Medical Devices Certificate of Registration for Medical Device Manufacture
Certificate of Registration for Medical Device Manufacture Written Notification for Sales and Lease of Controlled Medical Devices
Written Notification for Sales and Lease of Controlled Medical Devices
-

CHIDORI®
By coating specific areas of the blade tip of an electrosurgical knife, we have added two new functions: the ability to suppress carbide adhesion, and to control temperature in-crease.
-
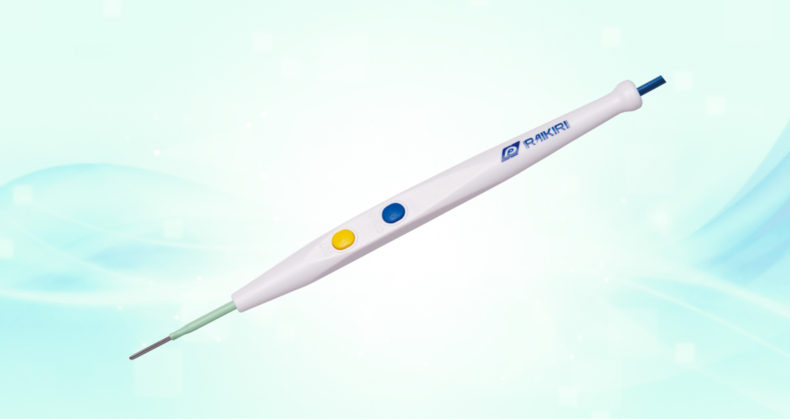
RAIKIRI®
Shaped to alleviate hand fatigue even in long-duration surgery, this scalpel is treated with a nonslip coating to promote ease of grip during surgery.
-
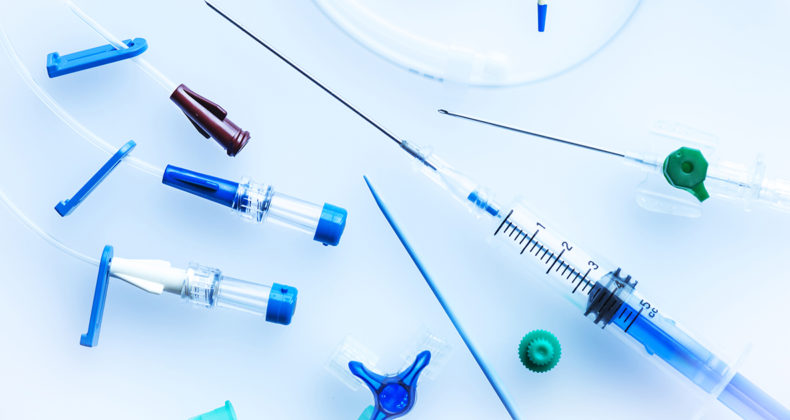
Surface Treatment for Medical Devices
We augment medical devices with surface coating by applying and drying chemical agents. Our coatings can be applied to a wide range of materials, including metals, glass, ceramics, and resins.
-

Utsunomiya Plant
Located within the precincts of Nihon Parkerizing’s Utsunomiya Site (Total Area: 46,492 m2). Established in 2018, it is currently equipped with a Cleanroom No. 1 and Cleanroom No. 2.
-

Contact
Your inquiry will be checked during regular business hours. For urgent inquiries, please contact us by telephone.

